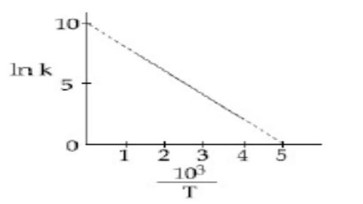
The rate constant (k) of a reaction is measured at different temperatures (T), and the data are plotted in the given figure. The activation energy of the reaction in kJ mol?¹ is:
The rate constant (k) of a reaction is measured at different temperatures (T), and the data are plotted in the given figure. The activation energy of the reaction in kJ mol?¹ is:
Option 1 -
2R
Option 2 -
2/R
Option 3 -
R
Option 4 -
1/R
-
1 Answer
-
Correct Option - 1
Detailed Solution:lnK = -E_a/RT + I
-E_a/R = slope is negative
⇒ -E_a/R = (10-0)/ (5-0)
E_a = 2R
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers