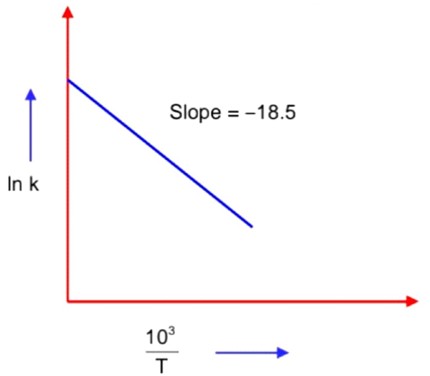
The rate constants for decomposition of acetaldehyde have been measured over the temperature range 700 – 1000 K. The data has been analysed by plotting ln k vs
graph. The value of activation energy for the reaction is ___________ kJ mol-1.
(Nearest integer)
(Given : R = 8.31 J K-1 mol-1)
The rate constants for decomposition of acetaldehyde have been measured over the temperature range 700 – 1000 K. The data has been analysed by plotting ln k vs graph. The value of activation energy for the reaction is ___________ kJ mol-1.
(Nearest integer)
(Given : R = 8.31 J K-1 mol-1)
-
1 Answer
-
Slope =
=> Ea = 18.5 × 1000 × 8.31 = 153.735 × 103 J = 154 KJ
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers