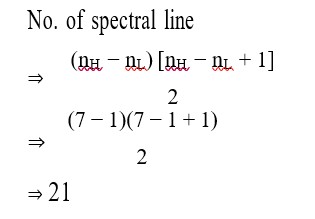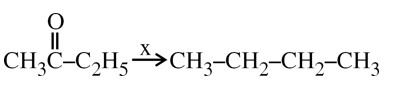The ratio of the mass percentages of 'C&H' and 'C&O' of a saturated acyclic organic compound 'X' are 4:1 and 3:4 respectively. Then, the moles of oxygen gas required for complete combustion of two moles of organic compound 'X' is [numerical value].
The ratio of the mass percentages of 'C&H' and 'C&O' of a saturated acyclic organic compound 'X' are 4:1 and 3:4 respectively. Then, the moles of oxygen gas required for complete combustion of two moles of organic compound 'X' is [numerical value].
Similar Questions for you
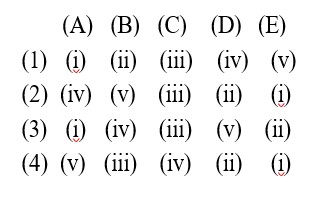
Kindly consider the solution
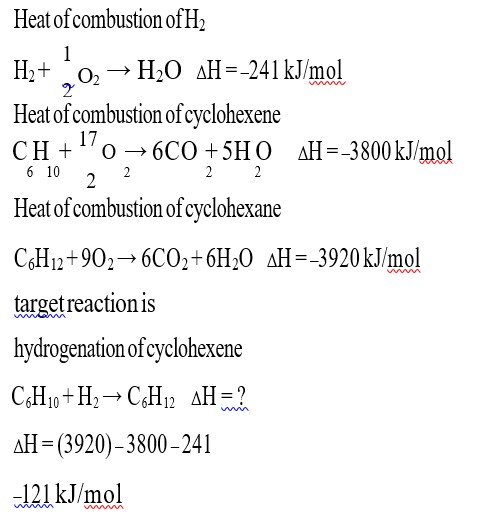
Fact.
No. of spectral line
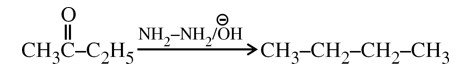
Wolf Kishner Reduction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers