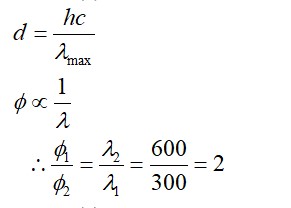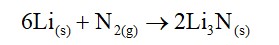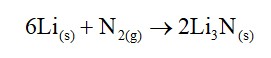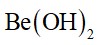The s-block elements are characterised by their larger atomic sizes, lower ionisation enthalpies, invariable +1 oxidation state and solubilities of their oxosalts.In the light of these features describe the nature of their oxides, halides and oxosalts.
The s-block elements are characterised by their larger atomic sizes, lower ionisation enthalpies, invariable +1 oxidation state and solubilities of their oxosalts.In the light of these features describe the nature of their oxides, halides and oxosalts.
This is a long answer type question as classified in NCERT Exemplar
Alkali metals are ionic in nature due to their larger size. They have +1 oxidation states. Alkali metals forms three types of oxides such as peroxides, superoxides and normal oxides. The basic character of normal oxides increases fro
Similar Questions for you
Li+ has the highest hydration enthalpy.
Hence it is most hydrated
Therefore, Correct order of hydrated radii is Cs+ < Rb+ < K+ < Na+ < Li+
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Ten 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering