The spin only magnetic moment value for the complex
is________ BM.
The spin only magnetic moment value for the complex is________ BM.
Asked by Shiksha User
-
1 Answer
-
Co has +2 oxidation state
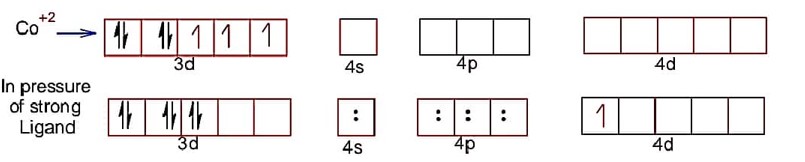
Hence, electronic configuration of CO2+ is . In complex given ligand CN is strong hence, after pairing in d-subshell, total number of unpaired electron =
spin magnetic moment =
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
