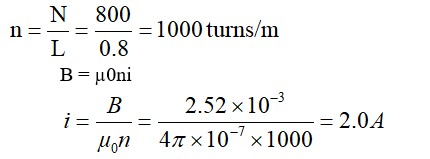The spin only magnetic moment value of an octhahedral complex among CoCl3.4NH3, NiCl2.6H2O and PtCl4.2HCl, which upon reaction with excess of AgNO3 gives 2 moles of AgCl is____________B. M. (Nearest integer)
The spin only magnetic moment value of an octhahedral complex among CoCl3.4NH3, NiCl2.6H2O and PtCl4.2HCl, which upon reaction with excess of AgNO3 gives 2 moles of AgCl is____________B. M. (Nearest integer)
-
1 Answer
-
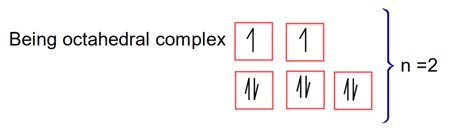
For precipitation of two moles of AgCl
Two Cl will produce as a free anion
CoCl3.4NH3 complex will Cl (will not give 2Cl)
complex will be H2 [PtCl6] will not any Cl
will produce two Cl ion.
precipitate formation
Similar Questions for you
Photodiode in reverse bias mode is used as intensity measuring device.
Tertiary haloalkane does not undergo SN2 reaction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers