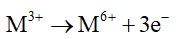The strength of an aqueous solution is most accurately determined by titrating : (Note : consider that an appropriate indicator is used)
The strength of an aqueous solution is most accurately determined by titrating : (Note : consider that an appropriate indicator is used)
Aq. in a burette and aqueous oxalic acid in a conical flask.
Similar Questions for you
After balancing change in oxidation state,
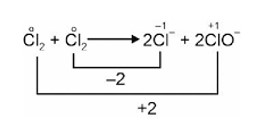
2Cl2 2Cl– + 2ClO–
Next, balance 'O' atoms,
2Cl2 +4OH–
Simplifying to get the simplest ratios,
Cl2 +2OH–
x = 1, y = 2, z = 1, p = 1
Disproportionation reaction is a reaction in which a substance (element) is simultaneously oxidised and reduced.
Potassium hydrogen phthalate is used to standardize NaOH solutions.
Phenolphthalein is used as an indicator to detect completion of titrations.
meq. of K2Cr2O7 = meq. of NO–2
n1 × 6 = 1 × 2
n1=1/3
Oxidation no. of a substance is proportional to the charge on it. In this case the charge is +
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 12th Chapter Eight 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering