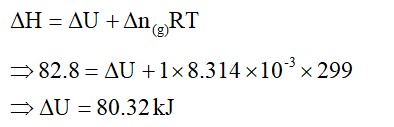The value of ∆fHᶱ for NH3 is – 91.8 kJ/ mol. Calculate enthalpy change for the following reaction :
2NH3(g) → N2(g) + 3H2(g)
The value of ∆fHᶱ for NH3 is – 91.8 kJ/ mol. Calculate enthalpy change for the following reaction :
2NH3(g) → N2(g) + 3H2(g)
-
1 Answer
-
This is a Short Answer Type Questions as classified in NCERT Exemplar
ΔH for formation is given. For the reverse reaction, ΔHchanges sign as the reverse of exothermic reaction will be endothermic. So, ΔH for decomposition is - (-91.8)=91.8 for one mole. But here, two moles are decomposing,
ΔH=2×91.8=183.6KJ
Similar Questions for you
Kindly go through the solution
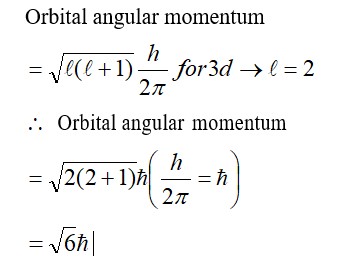
(1) [Ni (NH3)6]+2 → Ni+2 → d8, C. No. = 6,
SP3d2, Para
(2) [Co (H2O)6]+2 → Co+2 → d6, C. No. = 6
d2sp3, Dia
(3) [Ti (H2O)6]+3 → Ti+3 → d1, C. No. = 6
d2SP3, Para
(4) [Co (NH3)6]+3 → Co+3 → d5, C. No. = 6
d2sp3, Para
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers