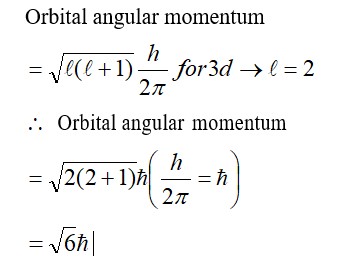
The volume of gas is reduced to half from its original volume. The specific heat will be ______.
(i) Reduce to half
(ii) Be doubled
(iii) Remain constant
(iv) Increase four times
The volume of gas is reduced to half from its original volume. The specific heat will be ______.
(i) Reduce to half
(ii) Be doubled
(iii) Remain constant
(iv) Increase four times
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (iii)
Specific heat capacity is the quantity of heat required to raise the temperature of one unit mass of a substance by one degree Celsius (or 1 Kelvin). That is why it is an intensive property which does not depend on mass.
Similar Questions for you
Kindly go through the solution
(1) [Ni (NH3)6]+2 → Ni+2 → d8, C. No. = 6,
SP3d2, Para
(2) [Co (H2O)6]+2 → Co+2 → d6, C. No. = 6
d2sp3, Dia
(3) [Ti (H2O)6]+3 → Ti+3 → d1, C. No. = 6
d2SP3, Para
(4) [Co (NH3)6]+3 → Co+3 → d5, C. No. = 6
d2sp3, Para
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Six 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering