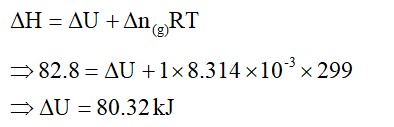Thermodynamics is not concerned about______.
(i) Energy changes involved in a chemical reaction.
(ii) The extent to which a chemical reaction proceeds.
(iii) The rate at which a reaction proceeds.
(iv) The feasibility of a chemical reaction.
Thermodynamics is not concerned about______.
(i) Energy changes involved in a chemical reaction.
(ii) The extent to which a chemical reaction proceeds.
(iii) The rate at which a reaction proceeds.
(iv) The feasibility of a chemical reaction.
This is a Multiple Choice Questions as classified in NCERT Exemplar
Thermodynamics is not concerned about how and at what rate these energy transformations are carried out but is based on initial and final states of a system undergoing the change. Laws of thermodynamics apply only when a system is in
Similar Questions for you
Kindly go through the solution
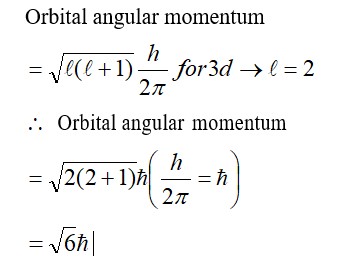
(1) [Ni (NH3)6]+2 → Ni+2 → d8, C. No. = 6,
SP3d2, Para
(2) [Co (H2O)6]+2 → Co+2 → d6, C. No. = 6
d2sp3, Dia
(3) [Ti (H2O)6]+3 → Ti+3 → d1, C. No. = 6
d2SP3, Para
(4) [Co (NH3)6]+3 → Co+3 → d5, C. No. = 6
d2sp3, Para
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Six 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering