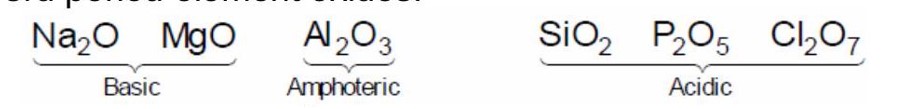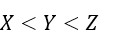Three elements X, Y and Z are in the 3rd period of the periodic table. The oxides of X, Y and Z, respectively, are basic, amphoteric and acidic. The correct order of the atomic numbers of X, Y and Z is:
Three elements X, Y and Z are in the 3rd period of the periodic table. The oxides of X, Y and Z, respectively, are basic, amphoteric and acidic. The correct order of the atomic numbers of X, Y and Z is:
On moving left to right in a period.
Acidic character of oxides is increase.
3rd period element oxides.
Similar Questions for you
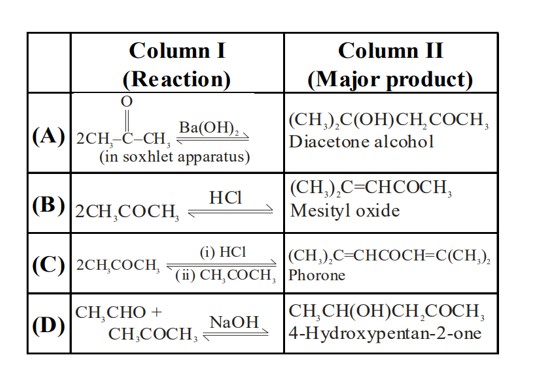
Yield of 4-hydroxypentan-2-one is low because aldol and diacetone alcohol are also formed.
On moving Left to Right along a period.
Atomic Radius → decreases.
Electronegativity → Increases.
Electron gain enthalpy → Increases.
Ionisation Enthalpy → Increases
Ionization enthalpy: B < Be < O < N
IE of N > O [Due to half-filled configuration]
IE of Be > B [Due to penetration effect]
Species =CH4
Electrons =101010
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Classification of Elements and Periodicity in Properties 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering