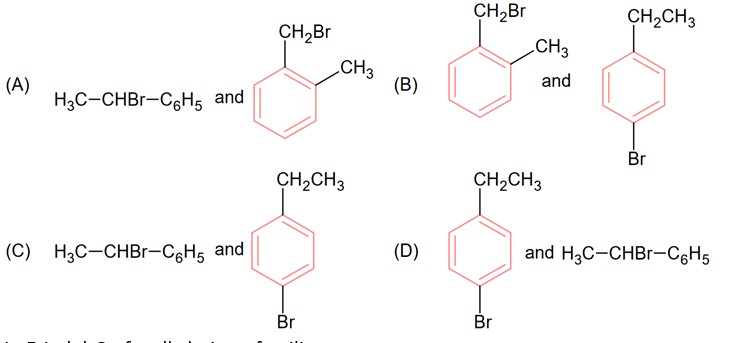
Two isomers (A) and (B) with molar mass 184 g/mol and elemental composition C, 52.2%; H. 4.9% and Br 42.9% gave benzoic acid and p-bromobenzoic acid respectively on oxidation with KMnO4. Isomer ‘A’ is optically active and gives a pale yellow precipitate when warmed with alcoholic AgNO3. Isomer ‘A’ and ‘B’ are respectively
Two isomers (A) and (B) with molar mass 184 g/mol and elemental composition C, 52.2%; H. 4.9% and Br 42.9% gave benzoic acid and p-bromobenzoic acid respectively on oxidation with KMnO4. Isomer ‘A’ is optically active and gives a pale yellow precipitate when warmed with alcoholic AgNO3. Isomer ‘A’ and ‘B’ are respectively
Option 1 -
a
Option 2 -
b
Option 3 -
c
Option 4 -
d
-
1 Answer
-
Correct Option - 3
Detailed Solution:Kindly consider the following figure
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers