Using crystal field theory, draw energy level diagram, write electronic configuration of the central metal atom/ion and determine the magnetic moment value in the following:
(i) [CoF6]3- ,[Co(H2O)6]2+ ,[Co(CN)6]3-
(ii) [FeF6]3- ,[Fe(H2O)6]2 + ,[Fe(CN)6]4 -
Using crystal field theory, draw energy level diagram, write electronic configuration of the central metal atom/ion and determine the magnetic moment value in the following:
(i) [CoF6]3- ,[Co(H2O)6]2+ ,[Co(CN)6]3-
(ii) [FeF6]3- ,[Fe(H2O)6]2 + ,[Fe(CN)6]4 -
-
1 Answer
-
This is a Long Answer Type Questions as classified in NCERT Exemplar
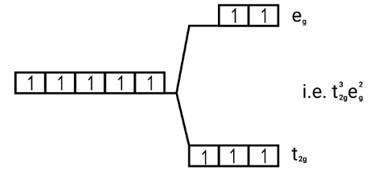
Ans: (i) Electronic cnfiguration: Co3+ =[Ar]3d6
Energy level diagram:
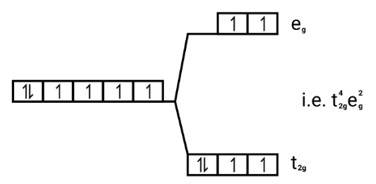
Magnetic moment:
Number of unpaired electrons (n)=4
Magnetic moment = μ= =
= = 4.9 BM
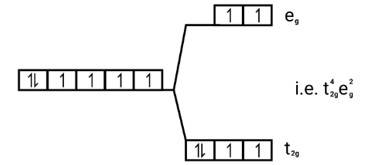
[Co(H2O)6]2+
Electronic cnfiguration: Co2+=[Ar]3 d7
Energy level diagram:
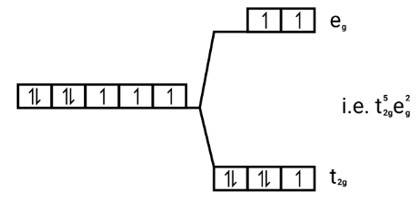
Magnetic moment: Since ,number of unpaired electrons (n)=3, therefore magnetic moment = = = 3.87BM
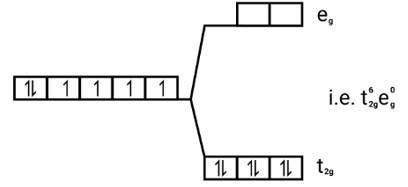
[Co(CN)6]3−
Electronic configuration: [Ar]Co3+=3 d6
Energy level diagram:
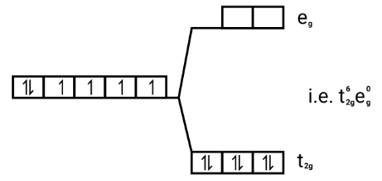 (ii)
(ii)Ans: [FeF6]3−
Electronic configuration: Fe3+=[Ar]3 d5
Ener
...more
Similar Questions for you
CoCl3.NH3 + AgNO3
x = 5
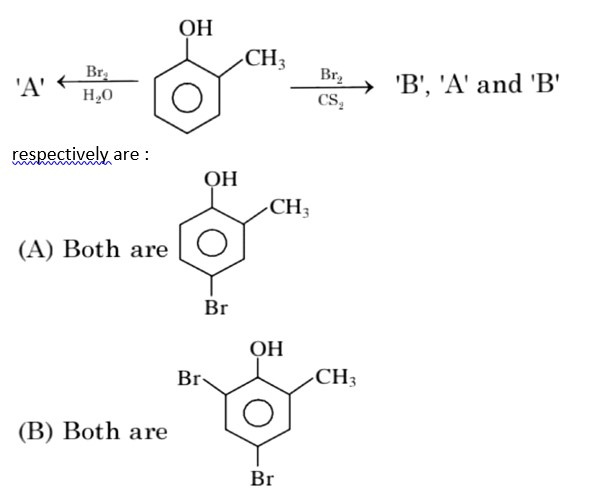
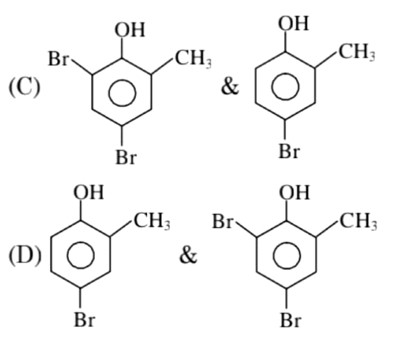
In H2O (polar solvent) dibromophenol derivative and in CS2 (non-polar solvent moneobromo phenol derivate is obtained.
3d => 4d => 5d CFSE increases for the same ligands.
Factual
⇒ leaching methods is used for those metal in which metal is more soluble than impurities and these are Al, Au, Ag, low grade Cu
σ bonded organometallic compound ⇒ M – C
σ-bond
and in π – bonded organo metallic compound
M – C
π bond
In ferrocene, there is π-bond
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers