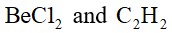What are the 5 postulates of VSEPR theory?
What are the 5 postulates of VSEPR theory?
Asked by Nishtha Datta
-
1 Answer
-
VSEPR theory predicts the shape of molecule based on postulate (assmptions). here are the important postulates as per the NCERT textbooks.
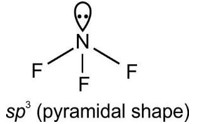
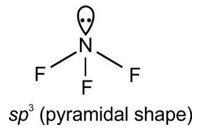
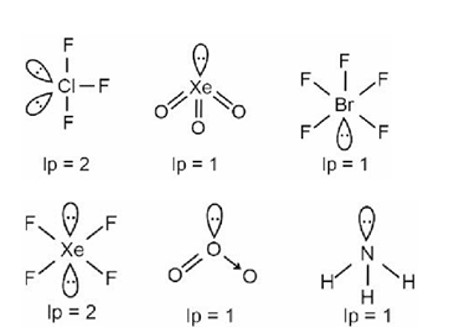
- The molecular geometry shape depends upon the number of valence shell electron pairs (bonded and non-bonded) around the central atom.
- The electron pairs (bonded and lone pairs) in the valence shell repel each other since their electron clouds are negatively charged.
- These electron pairs arrange themselves to minimize repulsion so that the molecule attains a stable structure with minimum energy.
- All electron pair repulsion doesn't repel each other equally, Electron pairs follows this order:
Lone pai
...more
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers