What do you understand by bond pairs and lone pairs of electrons? Illustrate by giving one example of each type.
What do you understand by bond pairs and lone pairs of electrons? Illustrate by giving one example of each type.
-
1 Answer
-
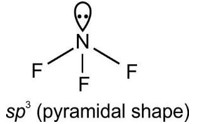
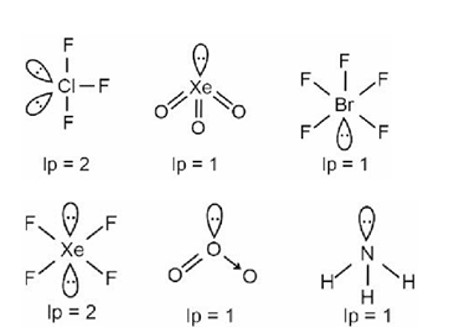
Molecules usually form chemical bond through either sharing or through rtransfering the electrons. During covalent bonding the electron pairs shared between atoms to form covalent bond are called shared pair or bond pair. At the same time, the electron pair which is not involved in sharing is called lone pair of electrons.
For example: CH4 has 4 bond pairs but H2O has 2 bond pairs and 2 lone pairs.

Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers