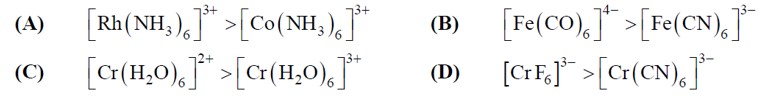What is the relationship between observed colour of the complex and the wavelength of light absorbed by the complex?
What is the relationship between observed colour of the complex and the wavelength of light absorbed by the complex?
-
1 Answer
-
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: When light wavelengths from a specific part of the spectrum are absorbed by a substance, the result is a complimentary colour. When a complex absorbs a wavelength of light, it reflects a complementary colour. If a violent colour is absorbed, for example, yellow is conveyed. The CFSE value, often known as the colour definer for any complex, comes next. To determine the wavelength value in order to determine which colour absorbs the most energy.
Δe=
As λ has shorter wavelength.
Low spin complexes absorb shorter wavelengths, while high spin complexes
...more
Similar Questions for you
CoCl3.NH3 + AgNO3
x = 5
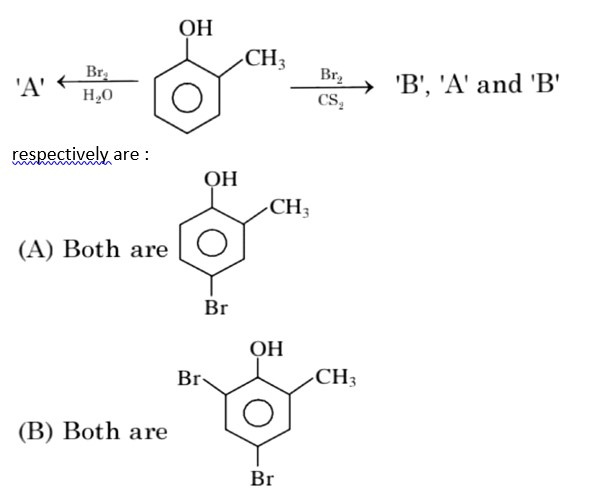
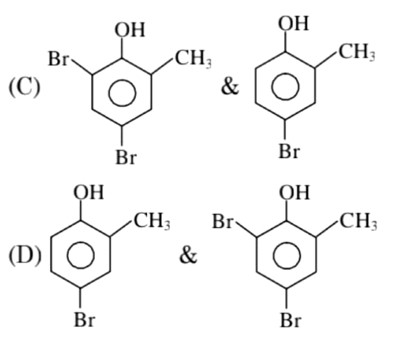
In H2O (polar solvent) dibromophenol derivative and in CS2 (non-polar solvent moneobromo phenol derivate is obtained.
3d => 4d => 5d CFSE increases for the same ligands.
Factual
⇒ leaching methods is used for those metal in which metal is more soluble than impurities and these are Al, Au, Ag, low grade Cu
σ bonded organometallic compound ⇒ M – C
σ-bond
and in π – bonded organo metallic compound
M – C
π bond
In ferrocene, there is π-bond
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers