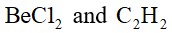What is VSEPR theory in class 11th Chemistry?
What is VSEPR theory in class 11th Chemistry?
-
1 Answer
-
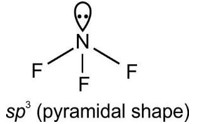
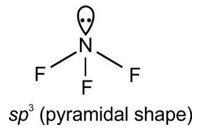
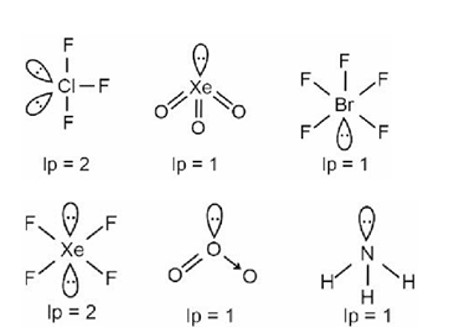
The Valence Shell Electron Pair Repulsion (VSEPR) Theory is an algorithm developed to predict the molecular geometry of the compounds. The VSEPR theory predict the molecular shape based on the repulsion between electron pairs (bonding and lone) around the central atom. As per the NCERT Textbooks:
“According to this theory, the shape of a molecule depends upon the number of valence shell electron pairs around the central atom. Electron pairs repel each other and try to remain as far apart as possible to minimise repulsion, thus determining the geometry of the molecule.”
You can use this theory in primarily explaining the molecular str
...more
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers