What kind of isomerism exists between [Cr(H2O)6]Cl3 (violet) and [Cr(H2O)5Cl]Cl2⋅H2O (greyish-green)?
A: Linkage isomerism
B: Solvate isomerism
C: Ionisation isomerism
D: Coordination isomerism
What kind of isomerism exists between [Cr(H2O)6]Cl3 (violet) and [Cr(H2O)5Cl]Cl2⋅H2O (greyish-green)?
A: Linkage isomerism
B: Solvate isomerism
C: Ionisation isomerism
D: Coordination isomerism
-
1 Answer
-
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: B
Hydrate isomerism is isomerism in which water is used as a solvent.
Coordination compounds with the same composition but varying ligand connectivity are known as linkage isomers.
Solvate isomerism has the same composition as free solvent but distinct solvent ligand molecules.
Except for the ligand that swaps places with the anion, ionisation isomers are identical isomers.
Coordination isomers are coordination compounds with distinct metal and ligand compositions.
Similar Questions for you
CoCl3.NH3 + AgNO3
x = 5
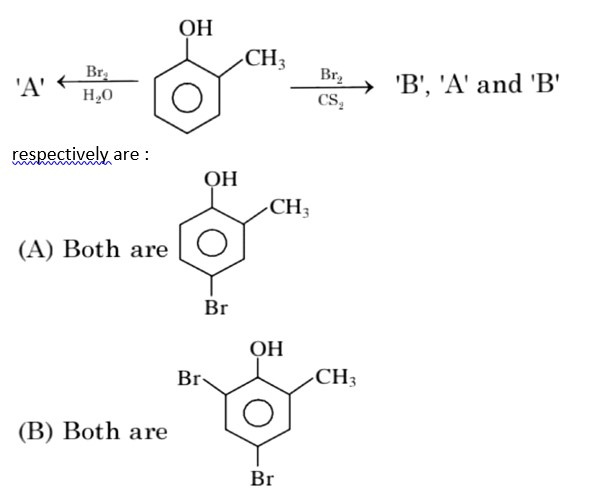
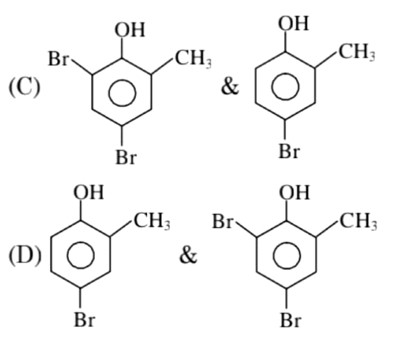
In H2O (polar solvent) dibromophenol derivative and in CS2 (non-polar solvent moneobromo phenol derivate is obtained.
3d => 4d => 5d CFSE increases for the same ligands.
Factual
⇒ leaching methods is used for those metal in which metal is more soluble than impurities and these are Al, Au, Ag, low grade Cu
σ bonded organometallic compound ⇒ M – C
σ-bond
and in π – bonded organo metallic compound
M – C
π bond
In ferrocene, there is π-bond
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers