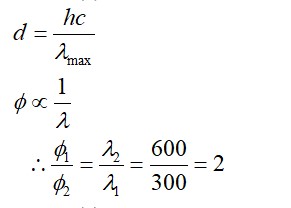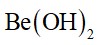When water is added to compound (A) of calcium, solution of compound (B) is formed. When carbon dioxide is passed into the solution, it turns milky due to the formation of compound (C). If excess of carbon dioxide is passed into the solution milkiness disappears due to the formation of compound (D). Identify the compounds A, B, C and D. Explain why the milkiness disappears in the last step .??????
When water is added to compound (A) of calcium, solution of compound (B) is formed. When carbon dioxide is passed into the solution, it turns milky due to the formation of compound (C). If excess of carbon dioxide is passed into the solution milkiness disappears due to the formation of compound (D). Identify the compounds A, B, C and D. Explain why the milkiness disappears in the last step .??????
This is a long answer type question as classified in NCERT Exemplar
Compound A reacts with water to form compound B. So, the compound A is calcium oxide. When water is added to calcium oxide, calcium hydroxide is formed. It is lime water. The compound gives a milky appearance which is compound C. The
Similar Questions for you
Li+ has the highest hydration enthalpy.
Hence it is most hydrated
Therefore, Correct order of hydrated radii is Cs+ < Rb+ < K+ < Na+ < Li+
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Ten 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering