Which acid of each pair shown here would you expect to be stronger?
(i) CH3CO2H or CH2FCO2H
(ii) CH2FCO2H or CH2ClCO2H
(iii) CH2 FCH2 CH2 CO2H or CH3 CHFCH2 CO2H
Which acid of each pair shown here would you expect to be stronger?
(i) CH3CO2H or CH2FCO2H
(ii) CH2FCO2H or CH2ClCO2H
(iii) CH2 FCH2 CH2 CO2H or CH3 CHFCH2 CO2H
-
1 Answer
-
(i) The +I effect of –CH3 group increases the electron density on the O-H bond. Therefore, the release of proton becomes difficult. On the other hand, the -I effect of F decreases the electron density on the O-H bond. Therefore, the proton can be released easily. Hence, CH2FCO2H is a stronger acid than CH3CO2H.
(ii) F has stronger -I effect than Cl (as fluorine is more electronegative than chlorine). Therefore, CH2FCO2H can release proton more easily than CH2ClCO2H. Hence, CH2FCO2H is a stronger acid than CH2ClCO2H.
(iii) Inductive effect decreases with increase in distance (the more will be the distance of the inductive group from
...more
Similar Questions for you
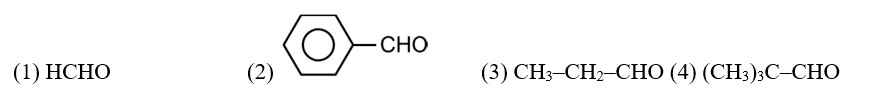
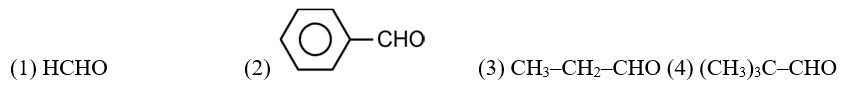
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Acetaldehyde (CH3CHO) gives positive lodoform test and positive Fehling's solution test
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers