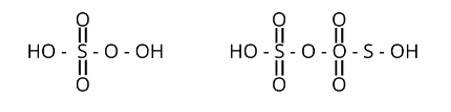
Which of the following are peroxoacids of sulphur?
(i) H2SO5 and H2S2O8
(ii) H2SO5 and H2S2O7
(iii) H2S2O7 and H2S2O8
(iv) H2S2O6 and H2S2O7
Which of the following are peroxoacids of sulphur?
(i) H2SO5 and H2S2O8
(ii) H2SO5 and H2S2O7
(iii) H2S2O7 and H2S2O8
(iv) H2S2O6 and H2S2O7
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i)
Peroxy linkage refers to the presence of a bond between oxygen and oxygen (O-O) in a molecule.
Only H2S2O6 and H2S2O7 of the sulphur oxoacids above have a peroxy linkage.
Similar Questions for you
HClO4 is the most acidic compound.
Group number = 11 (Atomic number = 111)
Heavier element of p block do not from pπ− pπ bonds as their atomic orbital are so large and diffius that they cannot have effecitve overlapping.
The inertness of
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering