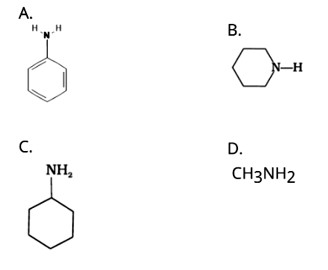
Which of the following is the weakest Brönsted base?
![]()
Which of the following is the weakest Brönsted base?
-
1 Answer
-
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (A)
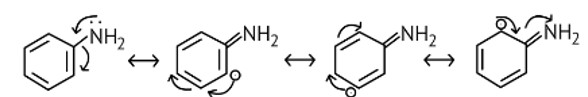
The NH2 group is directly attached to the benzene ring in aniline and other aryl amines. As a result, the unshared electron pair on the nitrogen atom is conjugated with the benzene ring, making it less available for protonation.
Similar Questions for you
In Amines, the nitrogen atom bonds with alkyl or aryl groups replacing hydrogen, whereas in amides, the nitrogen atom bonds directly with the carbonyl group (-CO-).
Kjeldahl's method is not applicable to compounds containing nitrogen in nitro and azo groups and nitrogen present in the ring.
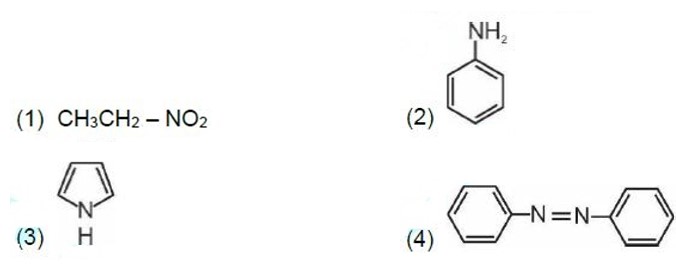
Correct order of basic strength in aqueous medium is
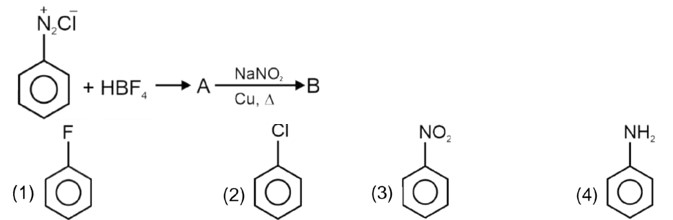
Kindly consider the following figure
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers