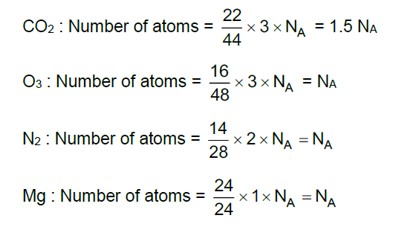Which of the following pairs have the same number of atoms?
(A) 16g of O2 and 4g of H2
(B) 16g of O2 and 44g of CO2
(c) 28g of N2 and 32g of O2
(D) 12g of c and 23g of Na
Which of the following pairs have the same number of atoms?
(A) 16g of O2 and 4g of H2
(B) 16g of O2 and 44g of CO2
(c) 28g of N2 and 32g of O2
(D) 12g of c and 23g of Na
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option(C), (D)
(A) The number of moles is given by the following formula,
Moles = -----------(1)
The number of moles of O2 is calculated by using equation (1) as follows,
Moles of O2 = = 0.5 mol
The number of atoms can be calculated
Similar Questions for you
In the medical entrance test NEET, there can be 1 to 3 questions from this chapter. Some year, the Chemistry section of NEET has only one question from this chapter and in some other years, there can be 3 questions.
The following are the key concepts of this chapter: Compound, Elements, Rules, Law of conservation of mass, Addition and Subtraction, Atomic Mass, Law of multiple proportions, and Molecular Mass.
As the name suggests, the first chapter of the NCERT Class 11 Chemistry introduces various basic concepts of chemistry, such as the definition and importance of chemistry, atomic matter and molecular masses, the mole concept, laws of chemical combination, empirical, stoichiometry, and molecular form
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter One 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering