Which of the following reactions of glucose can be explained only by its cyclic structure?
(a) Glucose forms pentaacetate.
(b) Glucose reacts with hydroxylamine to form an oxime.
(c) Pentaacetate of glucose does not react with hydroxylamine.
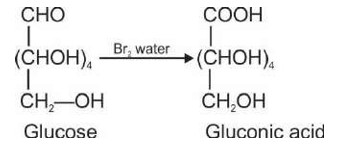
(d) Glucose is oxidised by nitric acid to gluconic acid.
Which of the following reactions of glucose can be explained only by its cyclic structure?
(a) Glucose forms pentaacetate.
(b) Glucose reacts with hydroxylamine to form an oxime.
(c) Pentaacetate of glucose does not react with hydroxylamine.
(d) Glucose is oxidised by nitric acid to gluconic acid.
-
1 Answer
-
This is a Multiple Answer Type Questions as classified in NCERT Exemplar
The Correct option is c.
The absence of a free (-CHO) aldehyde group is indicated by the fact that glucose pentaacetate does not react with hydroxylamine. The open structure of glucose cannot account for this, although the open chain structure of glucose can account for all other features.
Similar Questions for you
Insulin is a globular proteins.
Ionisation enthalpy increases in a period. Z dominates over screening effect (s) in a period as Zeff. increases.
Kindly go through the solution
Histidine is an essential amino acid
Lactose is a disaccharide which is formed by forming C? -C? glycosidic linkage between D-galactose and D- glucose.
Lactose - (Hydrolysis)-> D - galactose + D - glucose
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers