Which of the following species can act as the strongest base?
I. ΘOH
II. ΘOR
III. ΘOC6H5
IV. 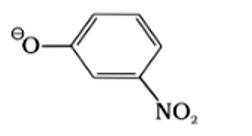
Which of the following species can act as the strongest base?
I. ΘOH
II. ΘOR
III. ΘOC6H5
IV. 
This is a multiple choice answer as classified in NCERT Exemplar
(II) ΘOR
The species which is the weakest acid has the strongest conjugate base. The ROH is the weakest acid among the given compounds due to the inductive effect of the alkyl group. Therefore, the strongest conjugate base is ΘOR.
Similar Questions for you
Bond is weaker then - bond.
Students while studying Coordnation Compound Chapter 5 must make a handy notes of formulas, definations, atomic number / weight, diagrams. These notes will be helpful in quick revision.
The ncert solutions for Class 12 Coordination Compound is updated on this artcile. Students can get the detailed solutions for intext and exericses here.
Students can refer the NCERT Solutions for Class 12 Chemistry Chapter 4 The d and f block Elements shared by the experts at Shiksha for NCERT Chemisytry textbook questions. The solutions for CBSE Chemistry d and f block questions is explanined in easy way for better understanding.
Students need to practice the Class 12 Chemistry previous year question to identify the important questions. Some of important questions are reactions of and in acidic, basic, and neutral medium, lanthanide contraction and its consequences, balancing redox reactions, etc.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 12th Chapter Eleven 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering
