Which of the following statements are correct about this reaction?
(i) The given reaction follows the SN2 mechanism.
(ii) (b) and (d) have the opposite configuration.
(iii) (b) and (d) have the same configuration.
(iv) The given reaction follows the SN1 mechanism.
Which of the following statements are correct about this reaction?
(i) The given reaction follows the SN2 mechanism.
(ii) (b) and (d) have the opposite configuration.
(iii) (b) and (d) have the same configuration.
(iv) The given reaction follows the SN1 mechanism.
-
1 Answer
-
This is a multiple choice answer as classified in NCERT Exemplar
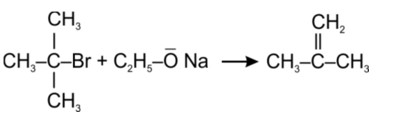
An SN2 reaction, also known as a nucleophilic substitution, is the reaction in question. The entering nucleophile causes the groups surrounding the carbon atom to migrate in the opposite direction of the nucleophile, causing the configuration of alkyl halides to invert. As a result, (b) and (d) will be in the opposite order. This reaction is also SN2 since there is just one phase in which OH- is added and Cl- is removed at the same time. (i) and (ii) are the right answers.
Correct Answer: Option (A) and (B)
Similar Questions for you
Photodiode in reverse bias mode is used as intensity measuring device.
Tertiary haloalkane does not undergo SN2 reaction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers