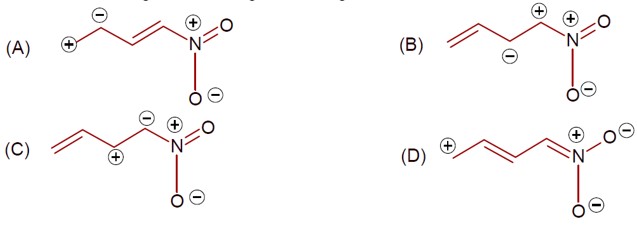Which one among the following resonating structure is not correct?
[Images of four resonance structures of an N-oxide compound]
Which one among the following resonating structure is not correct?
[Images of four resonance structures of an N-oxide compound]
Option 1 -
a
Option 2 -
b
Option 3 -
c
Option 4 -
d
-
1 Answer
-
Correct Option - 2
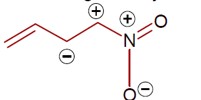
Detailed Solution:Same charge on adjacent atom is not stable. Hence the incorrect resonating structure is,
[Image of the incorrect resonance structure with adjacent positive charges]
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers