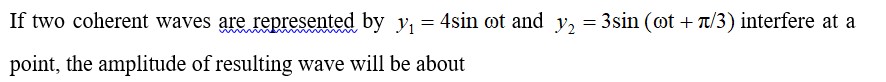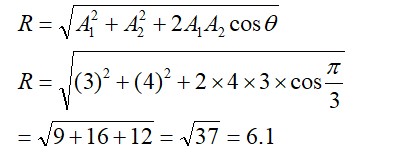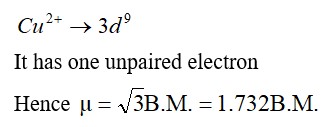Which one of the following species doesn't have a magnetic moment of 1.73BM. (spin only value)?
Which one of the following species doesn't have a magnetic moment of 1.73BM. (spin only value)?
Option 1 - <p>CuI</p>
Option 2 - <p><span class="mathml" contenteditable="false"> <math> <mrow> <mrow> <mo>[</mo> <mrow> <mi>C</mi> <mi>u</mi> <msub> <mrow> <mrow> <mo>(</mo> <mrow> <mi>N</mi> <msub> <mrow> <mi>H</mi> </mrow> <mrow> <mn>3</mn> </mrow> </msub> </mrow> <mo>)</mo> </mrow> </mrow> <mrow> <mn>4</mn> </mrow> </msub> </mrow> <mo>]</mo> </mrow> <mi>C</mi> <msub> <mrow> <mi>l</mi> </mrow> <mrow> <mn>2</mn> </mrow> </msub> </mrow> </math> </span></p>
Option 3 - <p><span class="mathml" contenteditable="false"> <math> <mrow> <msubsup> <mrow> <mi>O</mi> </mrow> <mrow> <mn>2</mn> </mrow> <mrow> <mo>−</mo> </mrow> </msubsup> </mrow> </math> </span></p>
Option 4 - <p><span class="mathml" contenteditable="false"> <math> <mrow> <msubsup> <mrow> <mi>O</mi> </mrow> <mrow> <mn>2</mn> </mrow> <mrow> <mo>+</mo> </mrow> </msubsup> </mrow> </math> </span></p>
2 Views|Posted 6 months ago
Asked by Shiksha User
1 Answer
V
Answered by
6 months ago
Correct Option - 1
Detailed Solution:
ln
ln
only one electron is unpaired in anti bonding MO,
Hence [n = 1 and
Similar Questions for you
K2Cr2O7 + H2O2 + H2SO4->
Potassium permanganate in alkaline medium oxidise lodide to lodate.
Compound A is
KMnO4 decomposes upon heating at 513 K and forms K2MnO4 and MnO2.
2KMnO4
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter One 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering