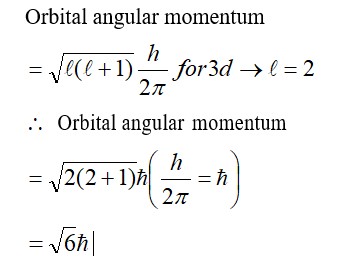
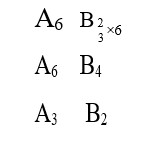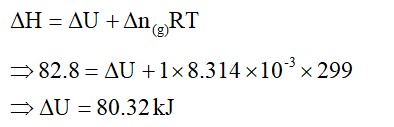Which quantity out of ∆rG and ∆rGᶱ will be zero at equilibrium?
Which quantity out of ∆rG and ∆rGᶱ will be zero at equilibrium?
-
1 Answer
-
This is a Short Answer Type Questions as classified in NCERT Exemplar
Gibbs energy for a reaction in which all reactants and products are in standard state. ΔrG° is related to the equilibrium constant of the reaction as follows
ΔrG = ArG° + RT In K
At equilibrium, 0 = ΔrG° + RT InA– ( {ΔrG = 0) or ΔrG° =-RT lnK
ΔrG° = 0 when K= 1
For all other values of K, ArG° will be non-zero.
Similar Questions for you
Kindly go through the solution
(1) [Ni (NH3)6]+2 → Ni+2 → d8, C. No. = 6,
SP3d2, Para
(2) [Co (H2O)6]+2 → Co+2 → d6, C. No. = 6
d2sp3, Dia
(3) [Ti (H2O)6]+3 → Ti+3 → d1, C. No. = 6
d2SP3, Para
(4) [Co (NH3)6]+3 → Co+3 → d5, C. No. = 6
d2sp3, Para
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers