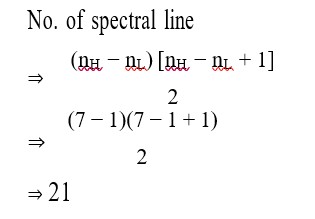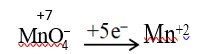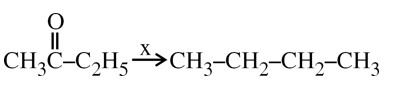While estimating the nitrogen present in an organic compound by Kjeldahl’s method, the ammonia evolved from 0.25 g of the compound neutralized 2.5 mL of M H2SO4. The percentage of nitrogen present in organic compound is _________.
While estimating the nitrogen present in an organic compound by Kjeldahl’s method, the ammonia evolved from 0.25 g of the compound neutralized 2.5 mL of M H2SO4. The percentage of nitrogen present in organic compound is _________.
-
1 Answer
-
Organic compound -> NH3
No. of millimoles of NH3 = 2 × no. of milimoles of H2SO4
= 2 × 2 × 2.5
= 10 milimoles
= No. of milimoles of N
Mass of nitrogen =
% of Nitrogen in compound =
= 56%
Similar Questions for you
Kindly consider the solution
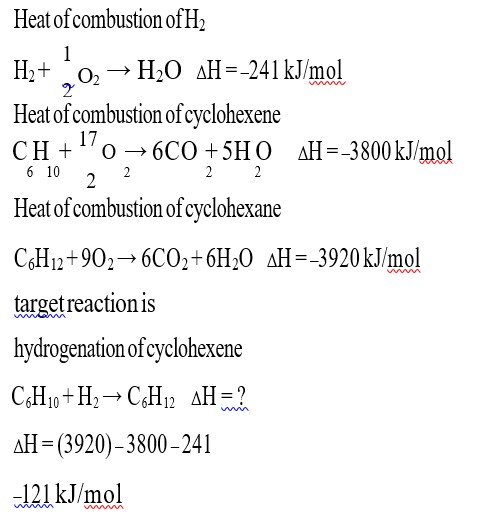
Fact.
No. of spectral line
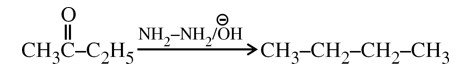
Wolf Kishner Reduction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers