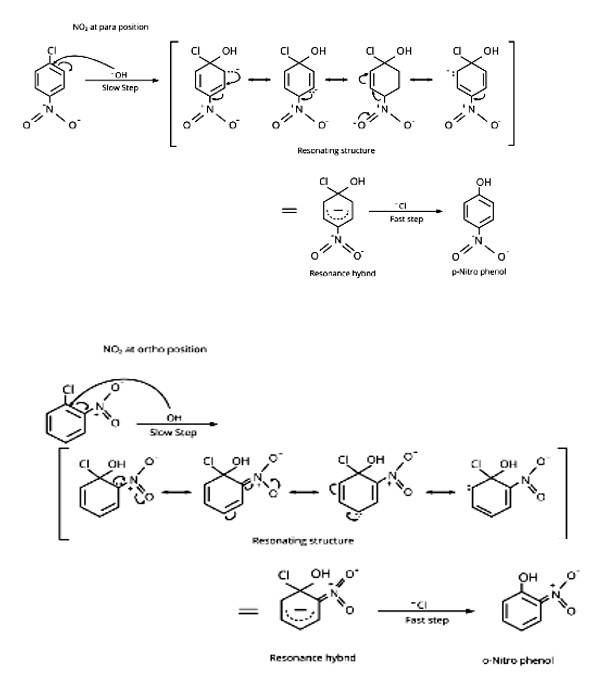
Why are aryl halides less reactive towards nucleophilic substitution reactions than alkyl halides? How can we enhance the reactivity of aryl halides?
Why are aryl halides less reactive towards nucleophilic substitution reactions than alkyl halides? How can we enhance the reactivity of aryl halides?
-
1 Answer
-
This is a long answer type question as classified in NCERT Exemplar
Because of the following reasons, aryl halides are less reactive towards nucleophilic substitution processes than alkyl halides:
(i) The halogen atom's electrons are conjugated to the aryl ring's -electrons, giving the C-X bond a partial double bond character. The whole molecule is then resonance stabilised, allowing for the formation of the following configurations. C-X bond cleavage in haloarenes is more difficult than in haloalkanes due to this bond creation.
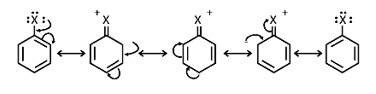
ii) The carbon linked to the halogen in haloalkanes is sp3 hybridised, whereas the carbon coupled to the halog
...more
Similar Questions for you
Photodiode in reverse bias mode is used as intensity measuring device.
Tertiary haloalkane does not undergo SN2 reaction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers