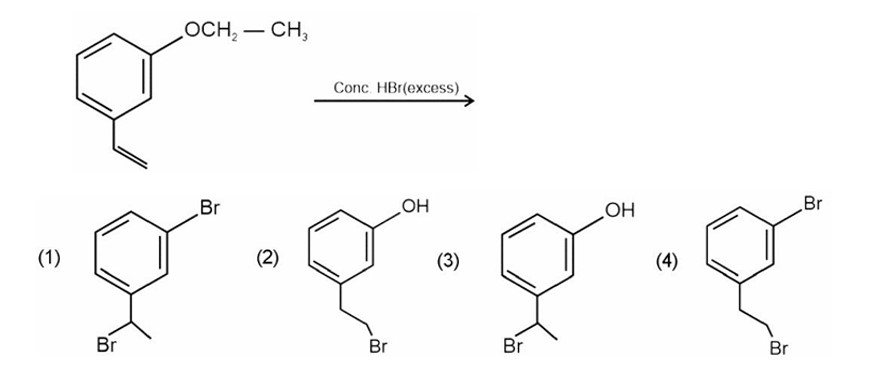
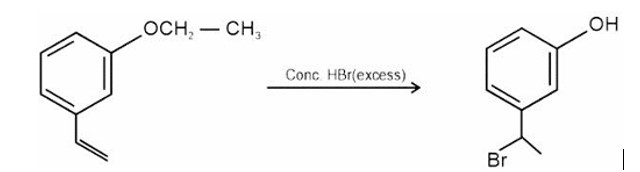
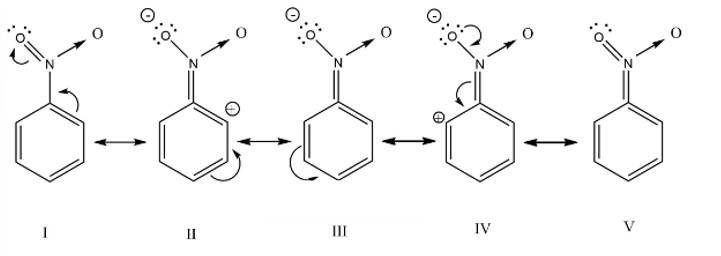
Why does presence of a nitro group make the benzene ring less reactive in comparison to the unsubstituted benzene ring. Explain.
Why does presence of a nitro group make the benzene ring less reactive in comparison to the unsubstituted benzene ring. Explain.
-
1 Answer
-
This is a short answer type question as classified in NCERT Exemplar
The nitro group strongly deactivate the benzene towards the electrophilic substitution reaction due to the -R and -I effect
Similar Questions for you
HBr adds to alkene in accordance with Markovnikov's rule.
Delocalisation of
To study Hydrocarbons for NEET, you can use the Hydrocarbons Class 11th NCERT solutions PDF.
Alkanes, Alkenes, Alkynes, and Aromatics hydrocarbons are the four main hydrocarbons.
Hydrocarbons are organic compounds made of only carbon and hydrogen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers