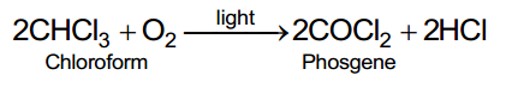
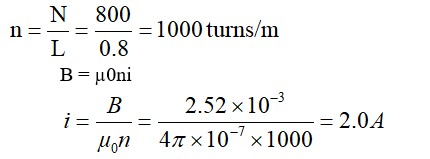Why is the solubility of haloalkanes in water very low?
Why is the solubility of haloalkanes in water very low?
-
1 Answer
-
This is a short answer type question as classified in NCERT Exemplar
Because energy is required to overcome the attractions between the haloalkane molecules as well as to break the hydrogen bonds between water molecules in order to dissolve a haloalkane in water, haloalkanes are only weakly soluble in water. New attractions between the haloalkane and the water molecules, on the other hand, release less energy since they are weaker than the water's initial hydrogen bonds.
Similar Questions for you
Photodiode in reverse bias mode is used as intensity measuring device.
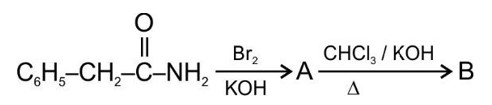
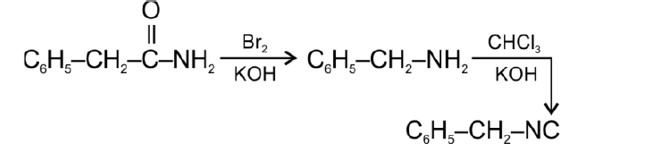
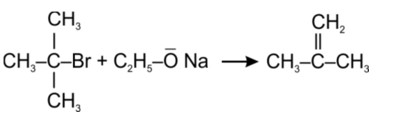
Tertiary haloalkane does not undergo SN2 reaction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers