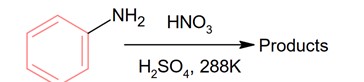
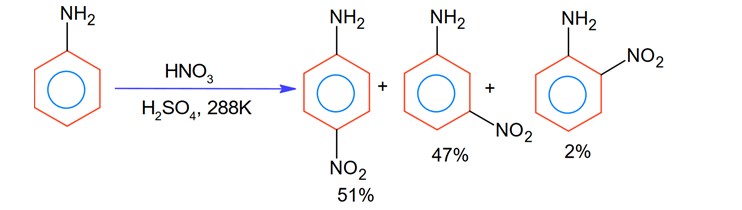
With respect to the following reaction, consider the given statements:
(A) o-Nitroaniline and p-nitoraniline are the predominant products.
(B) p-Nitroaniline and m-nitroaniline are the predominant products.
(C) HNO3 acts as an acid.
(D) H2SO4 acts as an acid.
Choose the correct option.
With respect to the following reaction, consider the given statements:
(A) o-Nitroaniline and p-nitoraniline are the predominant products.
(B) p-Nitroaniline and m-nitroaniline are the predominant products.
(C) HNO3 acts as an acid.
(D) H2SO4 acts as an acid.
Choose the correct option.
Option 1 -
(A) and (C) are correct statements.
Option 2 -
(A) and (D) are correct statements.
Option 3 -
(B) and (D) are correct statements
Option 4 -
(B) and (C) are correct statements.
-
1 Answer
-
Correct Option - 3
Detailed Solution:During nitration using HNO3 and H2SO4, H2SO4 act as an acid while HNO3 act as a base.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers