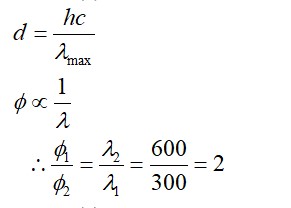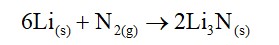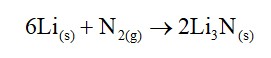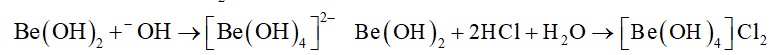Write Lewis strucure of O2 – ion and find out oxidation state of each oxygen atom? What is the average oxidation state of oxygen in this ion?
Write Lewis strucure of O2 – ion and find out oxidation state of each oxygen atom? What is the average oxidation state of oxygen in this ion?
This is a short answer type question as classified in NCERT Exemplar
The lewis structure of O2– ion is,
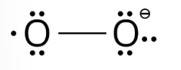
Oxygen atom having no charge has 6 electrons, so its oxidation number is zero. Oxygen atoms containing −1 charge have 7 electrons, so its oxidation number is −1. The average oxidation state of oxyg
Similar Questions for you
Li+ has the highest hydration enthalpy.
Hence it is most hydrated
Therefore, Correct order of hydrated radii is Cs+ < Rb+ < K+ < Na+ < Li+
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Ten 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering