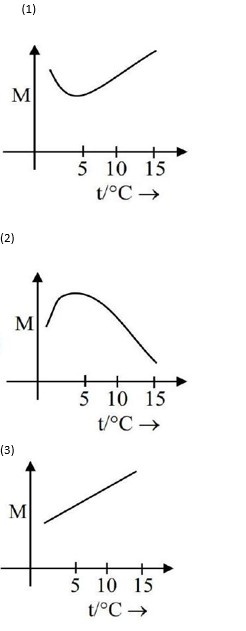
x g of NaCl is added to water in a beaker with a lid. The temperature of the system is raised from 1°C to 25°C. Which out of the following plots, is best suited for the change in the molarity ( M ) of the solution with respect to temperature? [Consider the solubility of NaCl remains unchanged over the temperature range]
x g of NaCl is added to water in a beaker with a lid. The temperature of the system is raised from 1°C to 25°C. Which out of the following plots, is best suited for the change in the molarity ( M ) of the solution with respect to temperature? [Consider the solubility of NaCl remains unchanged over the temperature range]
Option 1 -
a
Option 2 -
b
Option 3 -
c
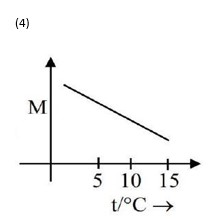
Option 4 -
d
Similar Questions for you
? Solvent is H? O, which is in excess
So using m ( molality ) = (x? ×1000)/ (x? × (M? )? )
? x? = 0.74 (Mol? = 18 g)
x? = 1 – 0.74 = 0.26 ∴ m = (0.26 × 1000)/ (0.74 × 18) = 19.5
ppm of O? = (wt. of O? ) / (wt. of H? O) × 10?
= (10.3 mg) / (1.03 × 10? mg) × 10?
= 10ppm
Molality = moles of solute / mass of solvent (kgs) ⇒ 1 = 0.5 / W (kgs)
Wsolvent (kgs) = 0.5 = 500 g
Molality = (mole of solute * 1000) / wt of solvent (gm)
100 = (n_solute * 1000) / [ (1 - n_solute) * 18]
(1 - n_solute) / n_solute = 1000 / (100 * 18) = 10/18
18 (1 - n_solute) = 10 n_solute
18 - 18 n_solute = 10 n_solute
18 = 28 n_solute
n_solute = 18 / 28? 0.6428 = 64.28 * 10? ²
Ans = 64 (Rounded off)
Let volume of solution = x ml
So mass of solution = 1.2x
And mass of water = x gm
Mass of solute = 0.2x
Molality = (W_solute * 1000) / (M_solute * W_solvent) = (0.2x * 1000) / (40 * x) = 5 m
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers