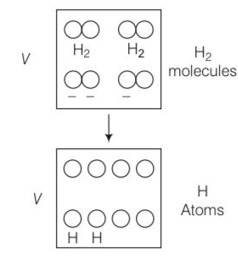
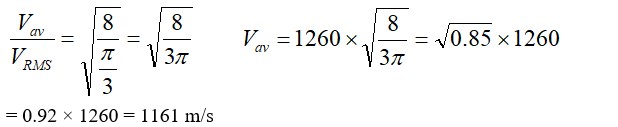1 mole of H2 gas is contained in a box of volume V = 1.00 m3 at T = 300K. The gas is heated to a temperature of T = 3000K and the gas gets converted to a gas of hydrogen atoms. The final pressure would be (considering all gases to be ideal)
(a) Same as the pressure initially
(b) 2 times the pressure initially
(c) 10 times the pressure initially
(d) 20 times the pressure initially
1 mole of H2 gas is contained in a box of volume V = 1.00 m3 at T = 300K. The gas is heated to a temperature of T = 3000K and the gas gets converted to a gas of hydrogen atoms. The final pressure would be (considering all gases to be ideal)
(a) Same as the pressure initially
(b) 2 times the pressure initially
(c) 10 times the pressure initially
(d) 20 times the pressure initially
This is a multiple choice answer as classified in NCERT Exemplar
(d) When number of molecules breaks then number of moles would become double.
P where n is the number of moles and T is temperature of ideal gas
As gases breaks number of moles becomes twcice of initial so n2 =2n1
= 20
p2=20p1
so 20 tim
Similar Questions for you
PV = nRT
->Pµn
->Ratio=
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

physics ncert solutions class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering