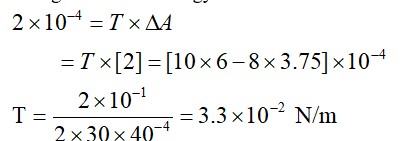12.1 Choose the correct alternative from the clues given at the end of the each statement:
(a) The size of the atom in Thomson's model is .......... the atomic size in Rutherford's model. (much greater than/no different from/much less than)
(b) In the ground state of .......... electrons are in stable equilibrium, while in .......... electrons always experience a net force. (Thomson's model/ Rutherford's model)
(c) A classical atom based on .......... is doomed to collapse. (Thomson's model/ Rutherford's model)
(d) An atom has a nearly continuous mass distribution in a .......... but has a highly non-uniform mass distribution in .......... (Thomson's model/ Rutherford's model)
(e) The positively charged part of the atom possesses most of the mass in .......... (Rutherford's model/both the models)
12.1 Choose the correct alternative from the clues given at the end of the each statement:
(a) The size of the atom in Thomson's model is .......... the atomic size in Rutherford's model. (much greater than/no different from/much less than)
(b) In the ground state of .......... electrons are in stable equilibrium, while in .......... electrons always experience a net force. (Thomson's model/ Rutherford's model)
(c) A classical atom based on .......... is doomed to collapse. (Thomson's model/ Rutherford's model)
(d) An atom has a nearly continuous mass distribution in a .......... but has a highly non-uniform mass distribution in .......... (Thomson's model/ Rutherford's model)
(e) The positively charged part of the atom possesses most of the mass in .......... (Rutherford's model/both the models)
12.1 The size of the atom in Thomson's model is no different from the atomic size in Rutherford's model.
In the ground state of Thomson's model, electrons are in stable equilibrium. While in Rutherford's model, electrons always experience a net force.
A classical atom based on Rutherford's model, is d
Similar Questions for you
Kindly go through the solution
Change in surface energy = work done
|DE0| = –10.2
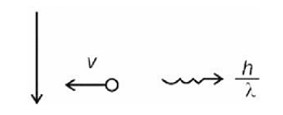
]
= 3 m/s
n = 4
Number of transitions =
Kinetic energy: Potential energy = 1 : –2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering