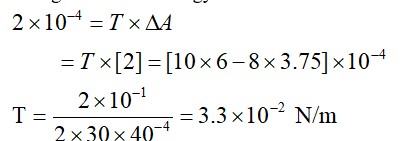12.15 The total energy of an electron in the first excited state of the hydrogen atom is about –3.4 eV.
(a) What is the kinetic energy of the electron in this state?
(b) What is the potential energy of the electron in this state?
(c) Which of the answers above would change if the choice of the zero of potential energy is changed?
12.15 The total energy of an electron in the first excited state of the hydrogen atom is about –3.4 eV.
(a) What is the kinetic energy of the electron in this state?
(b) What is the potential energy of the electron in this state?
(c) Which of the answers above would change if the choice of the zero of potential energy is changed?
12.15 Total energy of the electron, E = -3.4 eV
Kinetic energy of the electron is equal to the negative of the total energy
K = -E = 3.4 eV
Potential energy (U) of the electron is equal to twice the negative of kinetic energy
U = -2K = -6.8 eV
The potential energy of a system depends on the reference poi
Similar Questions for you
Kindly go through the solution
Change in surface energy = work done
|DE0| = –10.2
]
= 3 m/s
n = 4
Number of transitions =
Kinetic energy: Potential energy = 1 : –2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering