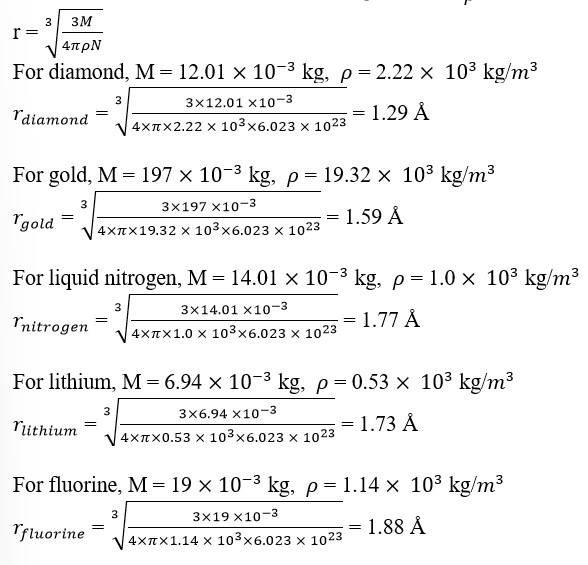
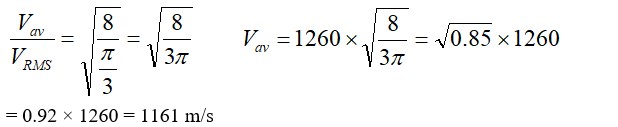13.14 Given below are densities of some solids and liquids. Give rough estimates of the size of their atoms:
Substance
Atomic Mass (u)
Density (
Carbon ( Diamond)
12.01
2.22
Gold
197.00
19.32
Nitrogen (liquid)
14.01
1.00
Lithium
6.94
0.53
Fluorine (liquid)
19.00
1.14
[Hint : Assume the atoms to be 'tightly packed' in a solid or liquid phase, and use the known value of Avogadro's number. You should, however, not take the actual numbers you obtain for various atomic sizes too literally. Because of the crudeness of the tight packing approximation, the results only indicate that atomic sizes are in the range of a few Å].
13.14 Given below are densities of some solids and liquids. Give rough estimates of the size of their atoms:
|
Substance |
Atomic Mass (u) |
Density ( |
|
Carbon ( Diamond) |
12.01 |
2.22 |
|
Gold |
197.00 |
19.32 |
|
Nitrogen (liquid) |
14.01 |
1.00 |
|
Lithium |
6.94 |
0.53 |
|
Fluorine (liquid) |
19.00 |
1.14 |
[Hint : Assume the atoms to be 'tightly packed' in a solid or liquid phase, and use the known value of Avogadro's number. You should, however, not take the actual numbers you obtain for various atomic sizes too literally. Because of the crudeness of the tight packing approximation, the results only indicate that atomic sizes are in the range of a few Å].
13.14 Let the atomic mass of a substance be = M and the density of the substance be =
Avogadro's number, N = 6.023
Volume of N number of molecules = ……. (i)
Volume of one mole of a substance = …… (ii)
Equating (i) and (ii), we get
Similar Questions for you
PV = nRT
->Pµn
->Ratio=
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

physics ncert solutions class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering