300 cal. Of heat is given to a heat engine and it rejects 225 cal. of heat. If source temperature is 227°C, then the temperature of sink will be__________°C.
300 cal. Of heat is given to a heat engine and it rejects 225 cal. of heat. If source temperature is 227°C, then the temperature of sink will be__________°C.
T1 = 227°C
= 500k
T2 =?
= 5 * 75
T2 = 375k
T2 = 102°C
Similar Questions for you
Process-AB Isobaric, &n
from newtan’s law of cooling :
Using average value :
Efficiency
-(i)
After the change :- -(ii)
--(ii)
At Resonance
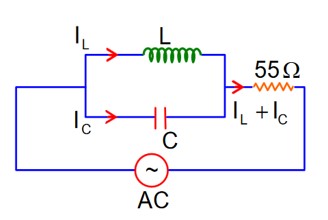
XL = XC
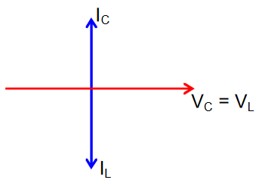
then lL = lC
Now phasor diagram
for L & C
So, Net current = zero
Therefore current through R circuit at resonance will be zero
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics NCERT Exemplar Solutions Class 12th Chapter Twelve 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering