A closed vessel contains 0.1 mole of a monatomic ideal gas at . If 0.05 mole of the same gas at is added to it, the final equilibrium temperature (in ) of the gas in the vessel will be close to
A closed vessel contains 0.1 mole of a monatomic ideal gas at . If 0.05 mole of the same gas at is added to it, the final equilibrium temperature (in ) of the gas in the vessel will be close to
2 Views|Posted 7 months ago
Asked by Shiksha User
1 Answer
Similar Questions for you
Process-AB Isobaric, &n
from newtan’s law of cooling :
Using average value :
Efficiency
-(i)
After the change :- -(ii)
--(ii)
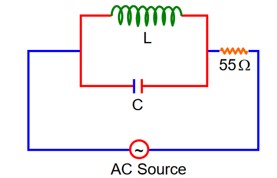
At Resonance
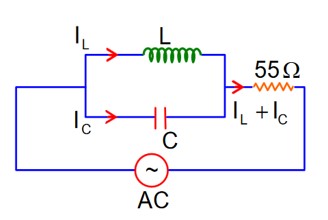
XL = XC
then lL = lC
Now phasor diagram
for L & C
So, Net current = zero
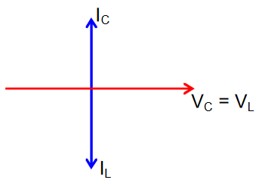
Therefore current through R circuit at resonance will be zero
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

Physics NCERT Exemplar Solutions Class 12th Chapter Twelve 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering