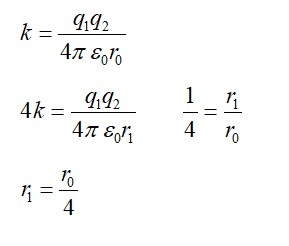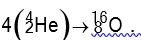A piece of wood from the ruins of an ancient building was found to have a 14 C activity of 12 disintegrations per minute per gram of its carbon content. The 14 C activity of the living wood is 16 disintegrations per minute per gram. How long ago did the tree, from which the wooden sample came, die? Given half-life of 14 C is 5760 yr.
A piece of wood from the ruins of an ancient building was found to have a 14 C activity of 12 disintegrations per minute per gram of its carbon content. The 14 C activity of the living wood is 16 disintegrations per minute per gram. How long ago did the tree, from which the wooden sample came, die? Given half-life of 14 C is 5760 yr.
This is a Short Answer Type Questions as classified in NCERT Exemplar
Explanation- R= 12dis/min per gm and R0= 16dis/min per gm T1/2= 5760yr
R=
By taking log
= log1016/12
= 2.303 (0.6020-4.771)5760/0.6931
= 2391.20yr
Similar Questions for you
Q = [4 *4.0026 – 15.9994] *931.5 MeV
Q = 10.2 MeV
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering