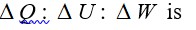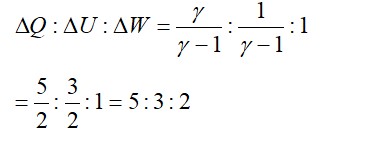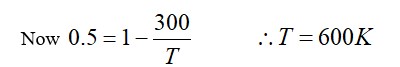Consider a Carnot's cycle operating between T1 = 500K and T2=300K producing 1 k J of mechanical work per cycle. Find the heat transferred to the engine by the reservoirs.
Consider a Carnot's cycle operating between T1 = 500K and T2=300K producing 1 k J of mechanical work per cycle. Find the heat transferred to the engine by the reservoirs.
This is a short answer type question as classified in NCERT Exemplar
Temperature of the source T1= 500K and sink T2= 300K
Work done W= 1000J
Efficiency of Carnot engine = 1-T2/T1= 1-300/500= 200/500= 2/5
Efficiency = W/Q1
So Q1= W/efficiency = 1000
Similar Questions for you
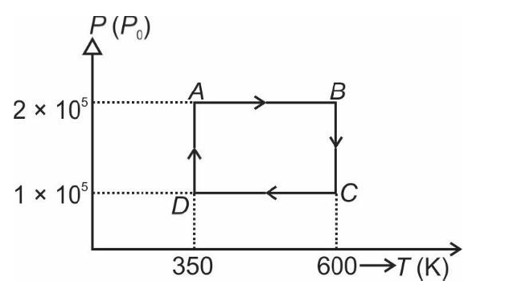
From A to B the process is isobaric
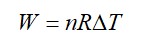
= W = 2 × R (600 - 350)
= 500 R
Heat is path dependent so path function but internal energy does not depend on path chosen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

physics ncert solutions class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering