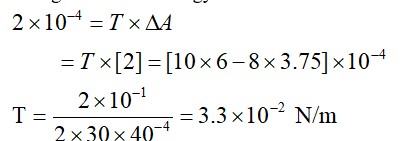Deutrium was discovered in 1931by Harold Urey by measuring the small change in wavelength for a particular transition in 1H and 2 This is because, the wavelength of transition depends to a certain extent on the nuclear mass. If nuclear motion is taken into account, then the electrons and nucleus revolve around their common centre of mass. Such a system is equivalent to a single particle with a reduced mass μ , revolving around the nucleus at a distance equal to the electron-nucleus separation. Here μ = meM / ( me + M ) where M is the nuclear mass and me is the electronic mass. Estimate the percentage difference in wavelength for the 1st line of the Lyman series in . 1H and 2H . (Mass of 1H nucleus is 1.6725 * 10-27kg , mass of 1H nucleus is 3.3374 * 10-27kg 3.3374 * 10-27 ?g , Mass of electron = 9.109 * 10-31 kg ).
Deutrium was discovered in 1931by Harold Urey by measuring the small change in wavelength for a particular transition in 1H and 2 This is because, the wavelength of transition depends to a certain extent on the nuclear mass. If nuclear motion is taken into account, then the electrons and nucleus revolve around their common centre of mass. Such a system is equivalent to a single particle with a reduced mass μ , revolving around the nucleus at a distance equal to the electron-nucleus separation. Here μ = meM / ( me + M ) where M is the nuclear mass and me is the electronic mass. Estimate the percentage difference in wavelength for the 1st line of the Lyman series in . 1H and 2H . (Mass of 1H nucleus is 1.6725 * 10-27kg , mass of 1H nucleus is 3.3374 * 10-27kg 3.3374 * 10-27 ?g , Mass of electron = 9.109 * 10-31 kg ).
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- total energy of electron
E=
The frequency hv=
=
= =
=
When me<
after solving we get 2.714
Similar Questions for you
Kindly go through the solution
Change in surface energy = work done
|DE0| = –10.2
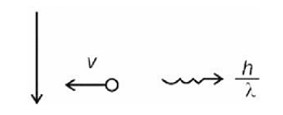
]
= 3 m/s
n = 4
Number of transitions =
Kinetic energy: Potential energy = 1 : –2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering