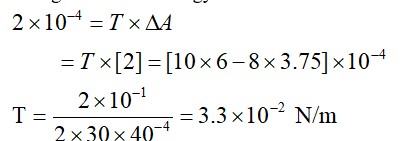For the ground state, the electron in the H-atom has an angular .momentum = h, according to the simple Bohr model. Angular momentum is a vector and hence there will be infinitely many orbits with the vector pointing in all possible directions. In actuality, this is not true,
(a) Because Bohr model gives incorrect values of angular momentum
(b) Because only one of these Would Have a minimum energy
(c) Angular momentum must be in the direction of spin of electron
(d) Because electrons go around only in horizontal orbits
For the ground state, the electron in the H-atom has an angular .momentum = h, according to the simple Bohr model. Angular momentum is a vector and hence there will be infinitely many orbits with the vector pointing in all possible directions. In actuality, this is not true,
(a) Because Bohr model gives incorrect values of angular momentum
(b) Because only one of these Would Have a minimum energy
(c) Angular momentum must be in the direction of spin of electron
(d) Because electrons go around only in horizontal orbits
This is a Multiple Choice Questions as classified in NCERT Exemplar
Answer – (a)
Explanation- In the simple Bohr model, only the magnitude of angular momentum is kept equal to some integral multiple of h/2 π, where, h is Planck's constant and thus, the Bohr model gives incorrect values of angular mome
Similar Questions for you
Kindly go through the solution
Change in surface energy = work done
|DE0| = –10.2
]
= 3 m/s
n = 4
Number of transitions =
Kinetic energy: Potential energy = 1 : –2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering