Given below are two statements:
Statement – I : When moment of an ideal gas undergoes adiabatic change from state to state then work done is w = where R = universal gas constant.
Statement – II : In the above case, when work done on the gas, the temperature of the gas would rise.
Choose the correct answer from the options given below:
Given below are two statements:
Statement – I : When moment of an ideal gas undergoes adiabatic change from state to state then work done is w = where R = universal gas constant.
Statement – II : In the above case, when work done on the gas, the temperature of the gas would rise.
Choose the correct answer from the options given below:
In case of adiabatic process
for adiabatic process :-
So, when work is done by the gas, temperature decreases and when work is done on the gas, temperature rises.
Similar Questions for you
Process-AB Isobaric, &n
from newtan’s law of cooling :
Using average value :
Efficiency
-(i)
After the change :- -(ii)
--(ii)
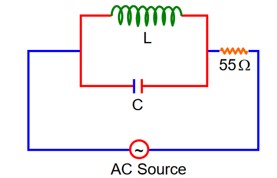
At Resonance
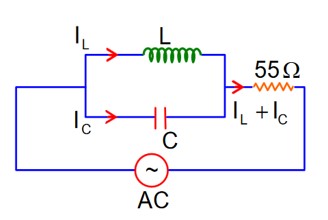
XL = XC
then lL = lC
Now phasor diagram
for L & C
So, Net current = zero
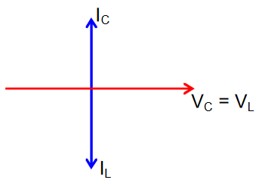
Therefore current through R circuit at resonance will be zero
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics NCERT Exemplar Solutions Class 12th Chapter Twelve 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering