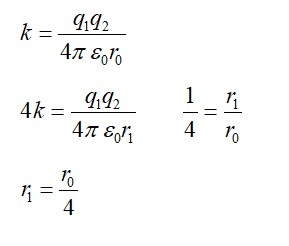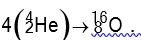How does the nuclear force overcomes repulsion between protons?
How does the nuclear force overcomes repulsion between protons?
-
1 Answer
-
As we all know that like charges repel each other. This means that there must be a repulsive electromagnetic force between positively charged protons. However, the nuclear force is said to be 100 times stronger than electromagnetic force which makes it strong enough to hold protons together within a nucleus. This nuclear force is much stronger than coulomb force at short ranges.
Similar Questions for you
Q = [4 *4.0026 – 15.9994] *931.5 MeV
Q = 10.2 MeV
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers