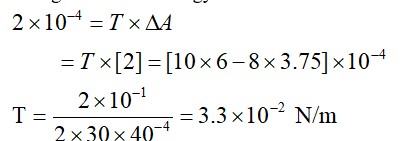If a proton had a radius R and the charge was uniformly distributed, calculate using Bohr theory, the ground state energy of a H-atom when (i) R = 0.1 Å and (ii) R = 10 Å .
If a proton had a radius R and the charge was uniformly distributed, calculate using Bohr theory, the ground state energy of a H-atom when (i) R = 0.1 Å and (ii) R = 10 Å .
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- in this type of situation centripetal force is balanced by electrostatic force
So mv2/r= -ke2/r2
According to bohr postuates
Mvr = h when n=1
On solving n
So after solving r= 0.51A0
So potential energy = -27.2eV , KE=
Similar Questions for you
Kindly go through the solution
Change in surface energy = work done
|DE0| = –10.2
]
= 3 m/s
n = 4
Number of transitions =
Kinetic energy: Potential energy = 1 : –2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering