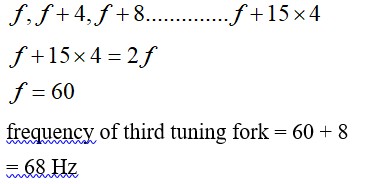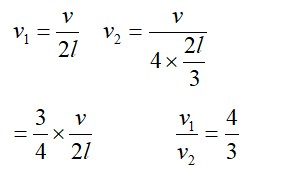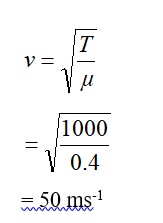If c is r.m.s. speed of molecules in a gas and v is the speed of sound waves in the gas, show that c/v is constant and independent of temperature for all diatomic gases.
If c is r.m.s. speed of molecules in a gas and v is the speed of sound waves in the gas, show that c/v is constant and independent of temperature for all diatomic gases.
This is a long answer type question as classified in NCERT Exemplar
we know that rms speed of molecules of a gas
C=
Where M is the molar mass of the gas
Speed of sound wave in gas v=
On dividing above equation we get
c/v =
c/v =
where = adiabatic constant for diatomic gas
c/v=constant
Similar Questions for you
The acceleration of wave is g/2. Its speed increases as it moves up. So answer is (2)
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics NCERT Exemplar Solutions Class 11th Chapter Fifteen 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering